| Updated: 08-Feb-2001 | NATO Information |
Click here
for
|
Depleted Uranium
1. Introduction
|
| Summary: | Depleted uranium is simply a waste product from the production of fuel rods for nuclear power plants and nuclear powered ships, as well as from the production of highly enriched uranium for nuclear weapons. |
Radioactive nuclei transform spontaneously into nuclei of another element.
Usually this process is accompanied by the emission of radiation. The
unit of measurement for radioactivity is the Becquerel (Bq). An activity
of 1 Bq means that one decay takes place per second. Different types of
atomic decay can be distinguished. In an Alpha-decay, the nucleus
emits an o> particle, consisting of two protons and two neutrons. In
a Beta-decay, the nucleus emits an electron, a so-called p-particle.
Alpha-, as well as Beta-decay, can be accompanied by gamma radiation,
a high energy electromagnetic radiation.
All three uranium isotopes discussed here emit a-particles. The
a-activity of natural uranium amounts to about 25'000 Bq per gram; that
of DU amounts to about 15'000 Bq per gram. Thus, the a-activity of DU
is about 40% less than that of natural uranium.
The newly formed nuclei resulting from the a-decay of uranium - called
daughter products - are not stable, but continue to decay, mostly by emitting
b-particles. The activity of its daughter products must be added to that
of uranium. The b-radiation of the daughter products of
natural uranium and DU have practically the same intensity, amounting
to about 25'000 Bq/g.
Together with its daughter products, depleted uranium has an activity
of approximately 40'000 Bq per gram. This means that about 40'000
decays take place per gram and per second. Only about 100 of these are
accompanied by high energy gamma radiation.
A comparison of the activities of a few radioactive materials:
| iodine 131 | 4'598'000'000'000'000 Bq/g |
| cesium137 | 3'206'000'000'000 Bq/g |
| Plutonium239 | 2'298'000'000 Bq/g |
| natural uranium with its daughter products ca. | 50'000 Bq/g |
| depleted uranium with its daughter products ca. | 40'000 Bq/g |
| Summary: | In comparison to other radioactive materials, neither natural uranium nor depleted uranium are particularly highly radioactive. |
In June 1998, the US Department of Energy (DoE) stored 734'000 metric
tons of uranium-hexafluoride. Two thirds of it - about 500'000 metric
tons - are depleted uranium; the rest is fluorine. Figures for the "stockpiles"
in other countries with enrichment facilities are not published. Together
they certainly store at least again as much DU. In 1995 as well as in
1996, about 35'000 metric tons of uranium were mined worldwide. From this
fact, it can be estimated, that yearly an additional 30'000 metric tons
can be added onto this already huge supply of DU.
With an usual hand monitor for the detection of radioactivity, a DU metallic
fragment can be detected from a distance of some 10 cm without any problem.
Since the alpha and beta radiation in the air have a very limited range
and only a small amount of gamma radiation is present, it is very difficult
to detect remains of DU ammunition from a distance of one meter or more.
Therefore, an efficient and extensive search for DU fragments is practically
impossible. A determination of the DU content in a soil sample or in the
dust of an air filter with the help of gamma detectors is barely possible
or even impossible. This makes it extremely difficult to establish a reliable
geographical distribution of the DU contamination on the former battlefields
in Iraq or Kosovo.
Reliable, quantitative information on DU contamination of the air or the ground must be determined in specially equipped laboratories. A special laboratory, such as AC-Laboratorium Spiez, can analyze between 10 and 100 samples per week, depending on the method of measurement.
Many civil applications of DU stem from its high specific weight
and its low price. Therefore, depleted uranium is used where maximum mass
in a limited volume is required, such as in counterweights in control
surfaces of wide-bodied aircraft. Also, because of its excellent shielding
properties for gamma radiation, it can be used in containers for spent
fuel rods from nuclear power plants.
In the military field, DU is used in armor and anti-armor ammunition. Incidentally, the use of depleted uranium has nothing to do with the use of uranium in its highly enriched form in atomic bombs!
Alloyed with 2% molybdenum or 0.75% titanium and after a special thermal treatment, uranium is as hard as hardened tool steel. Combined with its high density, it is a material well-suited for armor-piercing ammunition.
 |
| Fig 1: Armor-piercing ammunition shortly after being fired, at the moment of release from its sabot. |
According to the US technical literature, upon impact on armor a projectile made from DU keeps its form better than one made of tungsten or steel; the penetrator "sharpens" itself on impact, in contrast to the more expensive tungsten projectiles, which tend to mushroom. After penetrating the armor and as soon as the DU projectile again comes into contact with air, the part of DU, which is now in the form of a liquid or powder starts burning, thereby increasing its destructive effects. Often, this leads to setting the fuel tank on fire and/or detonating the ammunition stored in the tank.
In battle tanks, such as in the new version of the US Abrams, DU plates
are built in to improve the protective properties of the conventional
steel plate armor.
Because of the superiority of this type of ammunition, it has already been introduced by the military forces of several countries. The impressive results in the Gulf War may encourage more countries to procure DU-armor and -ammunition. By the way, Switzerland gave up the development of anti-armor ammunition containing radioactive material twenty years ago.
Depending on the use of DU, there are various ways that DU can enter
the environment and man, where it can possibly produce damaging effects.
One main exposure pathway is based on the fact that uranium is radioactive
and acts as a long-lasting source of radioactivity, irradiating man from
outside (external) the body. Depending on the uptake into the body
through the lungs (inhalation), with water and food through the digestive
tract (ingestion), or through wounds (inoculation), it will affect the
body from the inside (internal).
During normal operations in the industrial processing of DU, as
well as in accidents, the formation and emission of fine dust particles
(aerosols) are of immediate importance.
In fires, such as in DU ammunition storage facilities, DU burns
into a poorly soluble DU oxide powder. This contaminates the burning site
itself and is also partly carried into the air as a fine aerosol.
In the penetration through armor of high-speed ammunition, people can be injured by flying DU fragments. Depending on the material and thickness of the armor that is struck, a small portion of the DU is transformed into a fine aerosol, typically about 10% in the case of hard armor. The DU aerosol predominantly bums into poorly soluble uranium oxides, which can remain in the air at relatively high concentrations in closed spaces (tanks, bunkers) for quite some time. The portion that reaches the outside is quickly diluted, transported, and eventually deposited. The rest of the DU-projectiles, as well as those bullets that missed their targets, remain in the tank or in the nearby surroundings in the form of larger or smaller metal fragments.
In general, the chemical toxicity of a substance in the human
body is defined by a threshold concentration, below which no damage can
be observed.
The damaging effect of ionizing radiation is essentially a result
of the energy absorption by the body tissues, called radiation dose. The
unit of measurement for radiation doses is the Sievert (Sv), or the millisievert
(1 Sv = 1000 mSv). In order to relate the activity of a radioactive material
(in Bq) to the radiation dose it produces (in Sv), it must be known if
the body is irradiated internally or externally, which type of radiation
it is (alpha, beta, or gamma), and what energy the radiation has. The
ICRP (2) and other organizations have published corresponding
conversion factors for internal and external irradiation of man.
As in the case of chemical toxicity, a threshold dose also exists for ionizing radiation, below which no acute radiation damage can be observed. It is still unknown whether or not there is a threshold dose for the occurrence of late-time damages from radiation in the form of an increased probability of cancer. In order to avoid acute radiation damage and to limit late-time damages to a very low level, international recommendations and national laws for dose limits, as well as threshold values for the concentrations of artificial radioactive materials in the environment, air and food were established.
The damaging effect of DU, or otherwise expressed, its dangerousness
for man, is based on two of its properties:
Outside the body, DU only acts through irradiation. After incorporation,
chemical as well as radiological effects must be simultaneously taken
into consideration. The following review focuses on the situation on the
former battlefields of Iraq and the Balkans.
DU outside the body acts exclusively via the emitted gamma and beta radiation,
since the alpha-radiation is absorbed by the outermost layers of the skin
and therefore does not affect the living tissue.
The dose-rate from external radiation in the vicinity of DU is very low.
One kg DU at a distance of 1 m produces a dose of less than 1 mSv per
year. In comparison, an average person in Switzerland accumulates about
3 mSv per year from natural radiation sources.
According to some American publications, if a DU surface is touched by
the bare skin, a localized dermal dose of about 2 mSv per hour results.
The very improbable case of a direct contact for several days with the
same part of the skin would lead to a considerable dermal dose.
Radiation doses of millisieverts per year do not cause acute radiation damage; the only consequence would be a barely quantifiable increase in the risk of cancer.
| Summary: | In former battlefields, the inhalation of DU aerosols is the critical pathway for human exposure. An acute health risk is practically only to be feared from the chemical toxicity of uranium. |
Uranium in the form of fine particulate dust - such as that produced
in the uranium industry by mining uranium ore, or from the impact of high-speed
projectiles on armor, or from the burning of uranium - can enter the lungs
through inhalation. However, only about 25% of the particles with a diameter
smaller than 10 mrn are deposited in the lungs.
The decisive factor for the effect is the solubility of the inhaled
DU in the body fluids. American investigations have shown that after the
impact of DU projectiles on heavy armor, about 17% of the DU aerosol produced,
is found in an easily soluble form. In contrast, when DU burns in open
fires, practically no soluble uranium oxide is produced.
If uranium is in a chemically soluble form, the main part is relatively quickly eliminated - within days -through the bloodstream and the kidneys. At the same time, the kidneys are the target organ for the chemically-toxic effects of uranium, which can lead to impaired function or even total organ failure. A single inhalation of 8 mg uranium in soluble form is the threshold value for the occurrence of temporary kidney damage; while a level of 40 mg uranium in soluble form can lead to permanent kidney damage.
If the uranium is in a poorly soluble form, it can remain in the
lungs for a long time (years). The kidneys are hardly affected, since
the mobilized quantity of uranium is very small. In contrast, the radioactivity
of DU produces radiation doses in the lungs and bronchi. The ICRP calculates
a dose factor of 0.1 mSv/mg for the inhalation of poorly soluble 238U.
Therefore, except in extremely high uranium concentrations, acute radiation
damage is not expected.
An inhaled quantity of, e.g. 100 mg DU, would lead to permanent kidney
damage if in soluble form; if in insoluble form, it would lead to a slight
increase in the risk of cancer, about 0.04% in the worst case. Supported
by experiments in the US, it was calculated that the crew of a tank that
has been hit by DU-projectiles, could inhale up to 50 mg uranium aerosol.
This quantity could possibly lead to slight, reversible, toxicity-induced
damage to the kidneys, as well as to an internal radiation dose, roughly
equivalent to less than that of the yearly dose allowed for people professionally
involved in nuclear activities.
For people in open areas near destroyed tanks or near burning DU, the aerosol dose is considerably less. For people who entered the tanks or the vicinity of the former fire sites after the aerosol had settled, the internal contamination is also much much smaller. At the most, a smaller portion of the aerosol is resus-pended and could be inhaled. Overtime this danger is further reduced by climatic influences, such as rain or snow.
| Summary: | In former battlefields, the inhalation of DU aerosols is the critical pathway for human exposure. An acute health risk is practically only to be feared from the chemical toxicity of uranium. . |
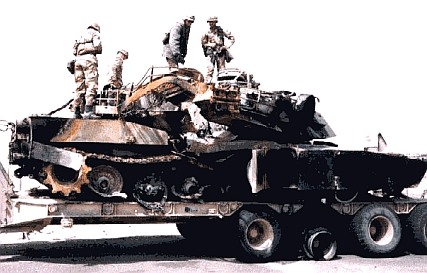 |
| Fig
2: American M1A1 tank having been hit by its own troops ("friendly fire incident") |
Only about 2 to 5 % of uranium in easily soluble form is taken up into
the bloodstream from the digestive tract. Thus, the threshold value for
the occurrence of reversible or permanent kidney damage after a single
exposure, is comparable to the one for the inhalation pathway. Uranium
in the form of uraniumoxide, which is poorly soluble in the body, is practically
not taken up by the digestive tract, and therefore, even in gram quantities,
has no chemically toxic effect.
Since the DU radiation dose through ingestion is orders of magnitude
less than that through the inhalation pathway, it is practically negligible.
| Summary: | The ingestion of DU poses practically no danger to the population in the former theatres of war. |
When DU is brought directly into the body through wounds, the toxicological
effect is also determined by the solubility of DU. Soluble uranium is
eliminated by the kidneys and can damage them when the threshold dose
is exceeded. Insoluble DU remains in the tissues for a long time, causing
a relatively high, locally limited radiation dose, leading to a slight
increase in the long-term risk of cancer. In the US, there is a group
of about 30 Gulf War veterans, victims of so-called "friendly fire
incidents" (Fig. 2), who live with DU fragments embedded in their
body. "Friendly fire" means they were mistakenly fired upon
by their own troops.
| Summary: | For the population in the former theatres of war wound contamination by DU has no significance. |
In Iraq, about 300 metric tons of DU ammunition were fired by American
and British troops. Recently, NATO confirmed the use of DU ammunition
in Kosovo battlefields, where approximately 10 metric tons of DU were
used.
The often-heard claim that this use of DU was a cheap way to "solve"
a waste problem, is certainly not true. The total quantity of DU in ammunition
that was used in Iraq and Kosovo corresponds to barely four days of DU
production worldwide.
Before the introduction of DU weapons in the US, estimates and calculations
led to the judgement that the occupants of tanks which have been hit by
DU projectiles and who survived these hits without great injury, may be
affected, at the most, by reversible, short-term effects on the kidneys,
as well as by an irradiation below the legal yearly limit. These risks,
in comparison to the other much greater risks during battle, were considered
to be acceptable. These estimates are rather conservative and appear to
be plausible. Medical examinations of a group of about 100 soldiers exposed
to DU aerosols in their own tanks from friendly fire incidents during
the Gulf War, have not discovered any health damages so far, that could
be attributed to DU. Somewhat less certain is the long-term prognosis
of adverse health effects for those injured by DU fragments. Up to now,
in this whole group of approximately 30 people, there has also been no
evidence of negative effects.
Additional and much larger groups of Gulf War veterans stayed in the
vicinity of the destroyed tanks and ammunition fires or entered such places
afterwards. In these cases, at the most, only rough estimates can be made,
since neither measurement data on DU emissions or dose calculations have
yet been published. One can estimate that the DU uptake by these groups
of people was far less than by those who were directly exposed. The well-known
health problems that were later observed in many Gulf War veterans, called
"Gulf War Syndrome" (3) cannot be explained
by exposure to uranium, and therefore, must be attributed to other causes.
Information on the occurrence of DU contamination during and after the
fighting is available neither for the Gulf Region nor the former Yugoslavia.
In the best case, a judgement can only be made in very broad terms.
In the vicinity of the impact point of DU ammunitions, it is not
excluded that individuals unaware of the contamination (e.g. children
playing with pieces of ammunition or in tank wrecks) and who stayed there
for an extended period of time, could have accumulated radiation doses
and/or could have incorporated uranium quantities exceeding the internationally
recognized limits. However, the probability that these quantities and
doses were so high that they led to acute illnesses, is very slim. Overlaying
the relatively high rate of naturally-occurring cancer, the additional
risk of cancer resulting from this radiation dose would be very small
and hardly detectable.
In all other places further away from the immediate battlefield
it is extremely improbable that people living there were afflicted by
health-threatening quantities of DU, through one of the contamination
pathways. In particular, the ingestion pathway is even less dangerous,
since uranium is only poorly transferred into the biological cycle "soil
- plants - animals -man". Evidence of damage to the genetic material
with an increase in abnormalities in newborns is not to be expected from
such very small DU doses.
The problem with the use of DU ammunition probably lies mainly in the fact that after the fighting, in the more highly contaminated places, the remaining local environmental contamination by uranium and its radiation exceeds the internationally recommended standards. However, one cannot directly conclude that there is a health risk for the people living there. Detectable extensive damage to the biosphere through DU contamination is very improbable, because the estimated concentrations are much too small.
"Depleted uranium - the apocalypse?" was the first question asked in the introduction. Based on the above presentation and the analysis of possible consequences on man and environment, the answer is as follows:
| An apocalypse caused by man as a result of the use of DU ammunition in Iraq and the Balkans is not worthy of discussion! |
Are there "irradiated Swiss soldiers in Kosovo?"
The answer to this second question is:
|
If certain minimal precautions are taken - i.e. no trespassing on tank wrecks and no long-term contact with remaining DU ammunition fragments - the health risks of a time-limited stay in a DU-contaminated area are shown to be negligibly small, especially in comparison to other risks such as mine fields, duds, snipers, etc. |
Military considerations
Up to now, the new ammunition and armor made from depleted uranium have
proven to be superior to previous systems. Thus, it would be difficult
for the military to abandon them. Furthermore, it is also clear that the
highly-praised superiority of these weapons will only last as long as
the opponent does not have them available too.
Views and Arguments of the Opponents
Various activist groups, especially in the US, Great Britain, and Holland,
are trying to involve international organizations in bringing about a
ban on DU ammunition. They are organizing information campaigns and symposiums
promoting their viewpoint that these weapons are "inhuman",
comparable to biological and chemical weapons.
Their few objective justifications for such a ban are derived from the
fact that the resident population has to continue to live on the former
battlefields, which according to valid radiation protection norms, are
at least locally radioactively contaminated; and from the fact that the
long-term effects of depleted uranium on man and the environment are not
entirely clarified.
View of the authors of this background information
Generally it is left to the reader to weigh and interpret this technical "Background Information" and the above summarized viewpoints. Clearly, this kind of ammunition leaves behind a long-lasting contamination on the battlefields, which is not compatible with civil radiation protection norms. This argument holds independently whether or not - objectively - there is a danger to man and the environment.
The Internet has proven to be a most extensive source of information.
A keyword search for "depleted uranium" produces about 10'000
citations on this topic. Among these are very good scientific papers on
the associated hazards and risks of depleted uranium, as well as on the
consequences of the use of DU ammunition in the Gulf War and Kosovo. Below
are some sources of information which the authors consider reliable.
http://www.rand.orq/publications/MR/MR1018.7/MR1018.7.html
http://www.gulflink.osd.mil/du/
http://www.antenna.nl/wise/uranium/
|
Furthermore the website of AC-Laboratorium Spiez, http://www.vbs.admin.ch/acls , contains a more comprehensive German version of this background information on DU, that can be downloaded by a "click" as a Word Document. |
The authors: E. Schmid,
Ch. Wirz
| AC-Laboratorium Spiez | Nuclear Issues and Weapon Effects | CH 3700 Spiez |